Most of the silver salts. Question 9 All of the following compounds are soluble in water EXCEPT.
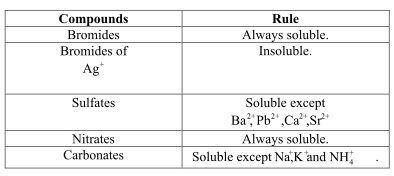
Predict Whether The Following Compounds Are Soluble Or Insoluble In Water Home Work Help Learn Cbse Forum
Douwdek0 and 2 more users found this answer helpful.

. K sp Ca 2I- 2 3O 2- 2. All nitrates are soluble no exceptions. Formaldehyde acetaldehyde and acetone all are water soluble.
Predict whether each of the following ionic compounds is soluble in water. Predict whether the following compounds are soluble or insoluble in water KNO3 CoBr2 AgNO3 AgBr BaSO4 NiCO3. Because alcohol is an all ready an a liquid.
C NH40 A Paz. D FeCl3 Eco 7 If you had an aqueous mixture that contained As K and Pb cations how many diff solids could precipitate if a chloride solution was added. The following substances are soluble in water except - 8393855 Keoutkopo Keoutkopo 10122020 Science Elementary School answered The following substances are soluble in water except aalcohol bsalt csoil dsugar 1 See answer cf050217 cf050217 Answer.
As an example methane is soluble in ethanol diethyl ether benzene toluene methanol acetone. Predict whether each of the following compounds is likely to. But except lead mercury and silver salts.
6 All of the following compounds are soluble in water EXCEPT. All of the following compounds are insoluble in water except. Which of the following equations is the solubility product for CaIO 3 2.
They do not dissolve in water. 6 All of the following compounds are soluble in water EXCEPT. K sp Ca 2 2 IO 3- c.
K sp Ca 2IO 3- d. K sp Ca 2I- 2 O 2- 6. Determine whether each of the following compounds is soluble or insoluble.
When any salt dissolved in waterwhether it is soluble or not can be determined using the solubility rule. If they do not dissolve in water those organic compounds are non-polar compounds. Y different A 1 B 2 C 3 D 4 E.
All of the following compounds are soluble in water except A. 2Salts containing are usually soluble in water. 1The salts having group I elements are soluble in water.
CaCL2 NH4Cl FeCl3 NaCl PbCl2. Some organic compounds are soluble only in organic solvents. Complete the following reaction and identify the Brønsted acidNaOHaq HClaq.
But insoluble in water 227 mg of CH 4 1 litre of water. View the full answer. Ammonium salts are also soluble in water.
C NH40 A Paz. All of the following silver compounds are soluble in water exceptAO AgClo4BO AgFCO Ag2SO4O AgNO3 Get the answers you need now. Sodium sulfate is among the soluble sulfates.
K sp Ca 2IO 3- 2. All carbonates are insoluble except those of NH₄ and group IA. All of the following compounds are soluble in water EXCEPT.
Tell whether the compound is. And since our barium is no exception here. So if we look at Adam the celebrity of the chloride atoms we can see that all the Clorox air soluble except the compounds off silver mercury and lead.
The following point comes under solubility rule.

Solubility Rules Chemistry Education Solubility Chemistry Classroom
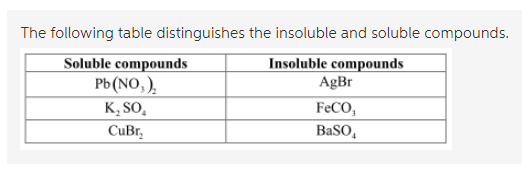
Predict Whether The Following Compounds Are Soluble Or Insoluble In Water Home Work Help Learn Cbse Forum

Predict Whether The Following Compounds Are Soluble Or Insoluble In Water Home Work Help Learn Cbse Forum
0 Comments